Matching Cancer Patients to Targeted Drugs: Two New Tools
A choreography of mutational events drives cancer cells to invade and metastasize, changing the biology in ways that enable the errant cells to resist treatments. While the traditional slash-and-burn approaches of chemotherapy and radiation attack rapidly-dividing cells, targeted treatments zero in on the altered proteins that reflect precise genetic changes in tumor cells. These are the somatic (“body”) mutations in just the affected cells, not the inherited mutations present in all of a patient’s cells.
Two new papers introduce tools to better match patients to targeted treatments or immunotherapies, based on interpreting the mutations behind a cancer’s initiation and spread. One is a machine learning tool called Cerebro, the other a scale for physicians to rank the evidence that a particular targeted treatment will work against a tumor with specific mutations.
A Brief History of Targeted Cancer Drugs
When my mother had breast cancer 30 years ago, her ordeal was both brutal and futile. She suffered through a one-size-fits-all kill-any-dividing-cell regimen of standard chemo drugs, then 5 years of tamoxifen. By blocking the overabundant estrogen receptors on her cancer cells that were enabling the hormone to rev up the cell cycle, tamoxifen was an early quasi-precision approach. But detecting extra estrogen and progesterone receptors, performed on all breast tumors, uses immunohistochemistry, not genetic testing.
My mother did what her doctors dictated. Back then, TV ads didn’t bombard cancer patients with the details of taxol, cisplatin, or Adriamycin.
In the 1990s came Herceptin. It targets a different receptor, HER2, that is extra abundant on the cells of 25 to 30 percent of breast cancers. Herceptin blocks human epidermal growth factor receptors (“HER”) from binding molecules that signal cell division. A cancer cell has 1 to 2 million receptors, a normal cell 20,000-100,000. (I told Herceptin’s story here).
On my breast cancer Facebook group, thousands strong, we introduce ourselves by name and receptor status: Jane Doe, ER+, PR+, HER2-.
An astonishing targeted cancer drug debut was Gleevec, which also blocks a signal to divide. The abstract in the April 5, 2001 New England Journal of Medicine reported that 53 of 54 patients with chronic myeloid leukemia responded. Gleevec is now used to treat several cancers, an early inspiration for the paradigm shift in tackling cancers by mutation, not location. Today “basket studies” test new drugs on cancers affecting different body parts but harboring the same mutations. The list of targeted therapies is growing.
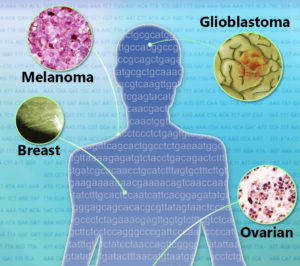
(Jonathan Bailey, NHGRI)
In 2017 came FDA approval for metastatic melanoma drugs Zelboraf and Tafinlar. They target a mutation, V600E, in the BRAF gene whose protein product is part of a signaling pathway that revs up cell division. Keytruda and Opdivo, also recent approvals, work another way, via an “immune checkpoint blockade” that inhibits molecules that in some cancers turn off the immune response. Keytruda and Opdivo attack a protein, PD-L1, which normally shuts off T cells, unleashing an immune response. Opdivo treats 12 types of cancer in combination with other treatments or as a last resort; Keytruda is currently approved just for non-small-cell lung cancer.
Direct-to-Consumer Advertising
Keytruda and Opdivo are familiar because of the maddening TV ads. An older gent points up at a skyscraper, beautiful wife by his side. Opdivo gave him “a chance to live longer!” Bristol-Myers Squibb initially received flak for omitting that “live longer” meant about 3 months, info now included in fine print. Keytruda has fared better than Opdivo because the clinical trial was restricted to patients in whom more than 50% of their cancer cells had excess PD-L1s. That’s important: choosing appropriate patients in clinical trials impacts demonstration of efficacy.
More recently, the shiny happy people approach in consumer advertising has come to metastatic breast cancer, with the 2017 FDA approval of Eli Lilly’s Verzenio. A mom watches her young daughter dance, two friends bake pies, a family blows soap bubbles near their garden, with nary a radiation marker or chemo port in sight. Small print reveals that progression is delayed but a survival benefit not yet demonstrated. Ads for Pfizer’s Ibrance offer equally delightful scenarios, but Novartis’ Kisqali actually asks real women how they feel. “I’m scared,” says one woman as another tears up. “When you find out you have metastatic breast cancer, you struggle.” The three drugs are CDK inhibitors, blocking part of the cell division cycle.
Beyond a Single Cancer Gene
Opdivo has about a 30% response rate. That’s okay for a cancer drug, but considering the multiple genetic pathways that drive cancer is a better way to stay ahead of the biological shifts that fuel treatment resistance. And more extensive genetic analysis is now feasible with costs of multi-gene test panels and exome and genome sequencing having plummeted.
 Like the ads for single-mutation targeting drugs, consumers are also in on genomic testing, in the form of ads such as those for Cancer Treatment Centers of America.
Like the ads for single-mutation targeting drugs, consumers are also in on genomic testing, in the form of ads such as those for Cancer Treatment Centers of America.
“Hundreds of laboratories in the United States provide NGS (next generation sequencing) cancer profiling, issuing tens of thousands of reports each year,” write Derrick Wood from Personal Genome Diagnostics at the Sidney Kimmel Comprehensive Cancer Center of Johns Hopkins University School of Medicine and colleagues in a new article in Science Translational Medicine. They point out that although these analyses conform to “CLIA” regulations (Clinical Laboratory Improvement Amendments), meaning they detect what they test for, they’re not yet FDA-approved as diagnostic tests to match cancer mutation profiles to specific drugs.
Still, the data from all this testing will ultimately translate into a much more tailored approach to treating cancer. The American Association for Cancer Research’s Genomics Evidence Neoplasia Information Exchange (Genie) and the “Multi-Center Mutation Calling in Multiple Cancers” part of the Cancer Genome Atlas are amassing much of the data.
A Bioinformatics Challenge
“In cancer, the genome is shot to hell,” a prominent researcher once told me. With hundreds of oncogenes and tumor suppressor genes that can mutate, each in a myriad of ways, plus bits of chromosomes that are jettisoned while others repeat repeat repeat, matching the “genomic profile” of a predominant cancer cell – a tumor may harbor genetically distinct cells – to a specific targeted drug becomes a problem of bioinformatics.
Imagine that the cancer cells of 30% of patients with mutation #1 respond to a drug. But 38% of patients with mutation #1 and mutation #2 in a different gene respond to the drug, as do 53% with mutations #1, #2, and #3, in three genes. It seems a matter of time before any mutational profile can be matched to at least one drug likely to stall disease progression.
To help analyze enough data to accurately sort the mutational wheat from the chaff, the Hopkins team turned to machine learning to invent Cerebro.
Better Than a Brain
In machine learning, algorithms train on real data to identify patterns that might be too complex or subtle for a mere human brain. Cerebro applies the random forest algorithm, which initially generates many random decision trees based on what’s already happened (real data on mutations and clinical responses) as well as “in silico” mutations that are theoretically possible based on a gene’s DNA sequence. Primed on the training data set, Cerebro then deduces which mutations in new data will be vulnerable to specific drugs.

The more data used in training the greater the predictive power. Given the huge datasets from all those folks having their cancer genomes probed, the time is right for this approach.
Cerebro trained on 30,000 mutations and 2 million errantly-called gene variants that didn’t actually predict drug response. They found that cells from an individual tumor harbor on average 267 somatic mutations and chromosome-level alterations, ranging from 1 to 5,871. Cancer is a changeling, cells differing genetically even within the same tumor.
After validation on large datasets, such as those from the Cancer Genome Atlas, Cerebro trumped human brains in matching new patients to targeted drugs. It considered not only what is known to exist, but what is possible, deduced from how a mutation alters the conformation of a protein and how that change affects the implicated biochemical pathway of carcinogenesis, invasion, or metastasis. Cerebro was more accurate in both directions. For example, the tool identified fewer meaningful mutations than standard techniques among a group of patients with non-small-cell lung cancer, but found more relevant mutations among patients with metastatic melanoma.
In the Meantime …
Until machine learning to quickly match cancer patient to optimal targeted treatment matures, another new paper, published in Annals of Oncology, introduces a scale that ranks the status of targeted drugs. It’s got a rather unwieldy name, ESCAT, for the ESMO (European Society for Medical Oncology) Scale for Clinical Actionability of Molecular Targets.
“Doctors receive a growing amount of information about the genetic make-up of each patient’s cancer, but this can be difficult to interpret for making optimal treatment choices. The new scale will help us distinguish between alterations in tumor DNA that are important for decisions about targeted medicines or access to clinical trials, and those which aren’t relevant,” explains Fabrice André, who originated the scale and is an oncologist at the Gustave Roussy Cancer Cancer in Villejuif, France.
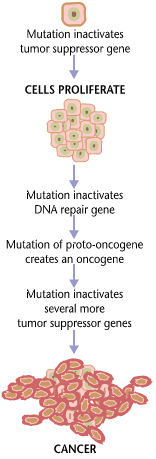 ESCAT provides six levels of clinical evidence to guide drug selection based on a tumor’s mutations:
ESCAT provides six levels of clinical evidence to guide drug selection based on a tumor’s mutations:
Tier I = “ready for implementation in routine clinical decisions” based on a clear survival benefit, such as Herceptin and Keytruda.
Tier II = “investigational targets that likely define a patient population that benefits from a targeted drug but additional data are needed.” (clinical improvement but no data yet on survival.)
Tier III = “clinical benefit previously demonstrated in other tumor types or for similar molecular targets.”
Tier IV = “preclinical evidence of actionability” (in human cells or animal models)
Tier V = “evidence supporting co-targeting approaches” (something else needed)
Tier X = “lack of evidence for actionability.”
Tier I mutations dictate drug choices. The scale will also enable oncologists to have meaningful discussions with patients desperate to try anything.
“ESCAT will bring order to the current jungle of mutation analysis so that we all speak the same language for classifying mutations and prioritizing how we use them to enhance patient care,” concludes André.
CODA
I never use the word “cure” in connection with cancer, but “NED” – “no evidence of disease” – seems too tentative. I’m hopeful that the patients taking targeted drugs today, along with frequent testing to identify new mutations in their cancer cells, are providing a future encyclopedia of knowledge that will facilitate selection of drugs to prevent progression, extend survival, or even restore a normal cell cycle.