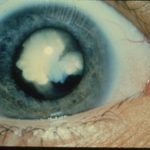
Cataracts and Amyloid Beta: Early Marker and New Drug Target?
I’ve just had cataract surgeries, so I wasn’t thrilled to find a new report in the Proceedings of the National Academy of Sciences comparing the protein glitch behind them to the one behind Alzheimer’s. Fortunately the similarity is only in how the proteins fold.

The amyloid beta buildup of cataracts could be an early marker that might eventually allow drug treatment to replace surgery. Too late for me, but that could be great news for the more than 18 million people worldwide blinded by cataracts because they can’t get surgery.
Cataracts develop as proteins coalesce in the lens, triggering spreading of patches of blurriness. They are the leading cause of blindness globally and affect half of people over age 50. My fellow PLOS blogger Hilda Bastian recently recounted the compelling story of the origin of cataract surgery.
A human lens is fascinating, in terms of biochemistry, cell biology, and evolution.
The Curious Anatomy of a Lens
An ocular lens is a feat of natural protein engineering that focuses light onto the retina, rather than scattering it. The lens is a transparent tiny dish that arises from cells stuffed with proteins called crystallins. These cells align in an indentation in the outer layer of a vertebrate embryo and elongate, smushing their dark-staining nuclei and other organelles to one end while clear, protein-packed cytoplasm gathers at the other.
The clear areas bend and form concentric layers that let light through. This illustration (from Victoria Webster, MUTAGENETIX, B. Beutler and colleagues, Center for the Genetics of Host Defense, UT Southwestern, Dallas, TX) shows the architecture of a human lens.
The cells of a lens remain in place for life. The organelles vanish in the eye’s center, no longer needed once the protein is made, leaving only transparent cytoplasm. A biological lens lacks blood to deliver hormones and neurotransmitters, but nutrients and growth factors seep in from the surrounding fluid. Cells at the periphery continue to divide and elongate, replenishing the lens.
More than 90% of the proteins that make up the lens are the crystallins. Also important are connexins, which monitor the crosstalk and exchange of materials between cells. A lens is a great example of chemistry becoming biology.
A lens should last a lifetime, but the human life span has extended quite a bit since our beginnings. That’s why so many of us eventually develop cataracts, a clouding of the lens that arises when the lens crystallins lose their solubility in water and become acidified. For me, that meant diminished night vision, a yellow-tinged world, difficulty telling dark colors apart, and tiny patches of blurriness in my visual field that for months I thought were due to smudged eyeglasses.
A Detour Into Evolution
Lens crystallins illustrate what paleontologist and science writer Stephen Jay Gould dubbed exaptation: a new function arising for a biological structure. Lens crystallins didn’t start out in eyes, but as proteins that protect cells from stress. In biology, stress means exposure to heat, light, and ultraviolet radiation from the environment and reactive oxygen species from the body (molecules with unpaired electrons that can damage biological structures).
Some lens crystallins, especially in birds, function as enzymes, particularly those that extract energy from nutrients in the absence of oxygen (anaerobic respiration), which happens in the eye. Perhaps proteins in primitive light-sensing structures of vertebrate ancestors that kept energy metabolism on track or countered stress in other ways began to aggregate, introducing a new function: focusing light rays. Vision improved, animals could hunt better, and over time, natural selection did its thing and eyes became more sophisticated. The multifunctional lens crystallins may have been “recruited” from pre-existing stress-induced enzymes to compensate for the absence of protective hormones or nerves in the eye.

The lens crystallins may in fact be more ancient than their presence in all vertebrates suggests. I first heard of them because they’re pretty much the same as the heat shock proteins of fruit flies, which enable the invertebrates to handle stress. (I was a Drosophila geneticist in a former life.)
The classic mechanism for a protein to take on a new function is by duplication of the gene that encodes it, and then elaboration of the new activity in the copy – like keeping a pair of favorite jeans while breaking in a new pair. And that’s what’s happened with the lens crystallins. The genes behind them bear duplications.
A Disorder of Protein Folding
Crystallin types are classified by size and charge. The alpha, beta, and gamma crystallins are in all vertebrates, delta only in birds and reptiles, and a few others found only in rabbits, guinea pigs, or frogs.
Mutations in the genes that encode the gamma crystallins in people cause rare inherited cataracts. But both inherited and acquired cataracts stem from crystallins becoming less water-soluble. One end of a molecule unfolds as the other becomes gummy.
Cataracts belong to a class of disorders that arise from misfolding of specific proteins. A quick review from biochem 101 is in order.
Proteins differ in their amino acid sequence – a clotting factor is not at all like a myosin – but they also differ in 3D shape, aka conformation. The amino acid sequence is the primary structure, the information encoded in the gene. Attractions between close amino acids in that sequence confer the secondary structure, and interactions between far-apart amino acids generate the looser folds of tertiary structure. Some proteins can fold up into different secondary structures even though they have the same amino acid sequence.

Cataracts arise from crystallin proteins that can contort into different versions of a common secondary structure called a beta-pleated sheet. By around the age of 40 in a future cataract patient, the crystalline beta-pleated sheets begin to compress themselves into an “overlapping zigzag” conformation called amyloid beta.
Sound familiar?
Yes, amyloid beta is the major misfolded protein associated with Alzheimer’s disease. Other disorders of protein folding include Parkinson’s, Huntington’s, and the “transmissible spongiform encephalopathies” (TSEs) like kuru, mad cow disease, fatal familial insomnia, and Creutzfeldt-Jakob disease. “Transmissible” means that the gummy protein form converts others, spreading the stickiness.
A New View of Cataracts
Ariel Alperstein, a chemist at the University of Wisconsin-Madison and colleagues, knew that in glassware under acidic conditions, lens crystallins aggregate into amyloid beta sheets and extend, forming fibrils. Most imaging techniques only pick up the fibrils, so they haven’t provided clues to the genesis and earlier stages of a cataract. But two-dimensional infrared (2DIR) spectroscopy can detect vibrations of the packed amyloid at an earlier stage.
The researchers turned 2DIR to the amyloid in 3 clear eyes from teens, 4 from adults (aged 44-69) who didn’t have cataracts, and 3 from older adults who had cataracts, all from the Lion’s Eye Bank of Wisconsin. Using whole eyes provided a more realistic view than past investigations that used torn lenses from patients having surgery.
“By using intact lens tissue and juvenile controls, our study provides evidence that cataracts may be an amyloid disease, akin to Alzheimer’s, type 2 diabetes, and other diseases with different manifestations, but all linked to amyloid formation,” the researchers write.
About 1% of the protein in the teen eyes was amyloid beta sheets, compared to 43% for the cataract eyes. But most telling were the adult eyes that didn’t have cataracts. They varied a lot in amyloid sheet content, whereas the teens were similar to each other as were the older eyes with cataracts. That variability suggests that percentage of aberrant amyloid beta could be an early marker of the condition.
The researchers also conducted a clever experiment. They examined lenses from eyes of a 14-year-old, a 59-year-old without cataracts, and a 65-year-old with cataracts, before and after exposing the eyes to UV light. UV chops up crystallins and hastens amyloid formation.
Before the UV exposure, the lenses from the teen and the adult without cataracts had less than 0.1% amyloid beta, but the 65-year-old eye with cataract had 7.4%. After 48 hours of UV, all of the lenses had more amyloid, and the amount in the lens with cataract had quadruped! Two months later, the teen lens hadn’t added amyloid, indicating that the aggregation stopped when the UV exposure stopped. That finding clearly links the protein buildup of cataracts to sun exposure, the researchers write.
2DIR envisioned down to 4 or 5 amyloid beta strands, compared to transmission electron microscopy, which only picks up fibrils that form from 50 or more of the sheets. That is, 2DIR glimpses an early stage in the amyloid buildup behind cataracts. And that’s clinically important because drugs in development target earlier stages and might one day replace surgery, enabling the prevention of blindness in millions. Lanosterol is one drug, which reverses protein aggregation in cataracts in rabbits, dogs, and human cells, and small molecules have been tested in mice.
In the developed world we tend to take cataract surgery for granted. Everybody told me not to worry, it was nothing. But for millions of others, the blurred patches grow and the world continues to be bathed in shades of yellow and brown, until vision fades permanently. I hope that the new view of cataracts as an amyloid disease will lead to simpler, cheaper, and more widely available treatment for the 50 million people that the National Eye Institute projects will have cataracts by 2050.