Probing Polar Bodies to Pick Disease-Free Embryos

After writing eleven editions of a human genetics textbook, I automatically assign chapter numbers to exciting new findings. But the 3-page case report in this week’s JAMA Neurology on selecting disease-free embryos tangled up my brain with all its connections.
The case report delves into:
a. Meiosis, including polar bodies
b. Mendel’s first law
c. Embryology
d. Prions
e. Protein folding
f. Assisted reproductive technologies (ARTs)
g. Neurogenetics
h. Bioethics
i. All of the above
The researchers (Alice Uflacker and Murali Doraiswamy from the Duke Institute for Brain Science, Svetlana Rechitsky and Ilan Tur-Kaspa from the Reproductive Genetics Institute in Chicago, and Michael Geschwind and Tricia See from UCSF) describe using ARTs to enable a woman whose relatives have a devastating and very rare brain disease to have children free of the family legacy.
AN INHERITED PRION DISEASE
The disease, Gerstmann-Straussler-Scheinker Syndrome (GSS), affects only 1-10 per 100 million births. Much of what’s known about it comes from an 8-generation family from Indiana that has had 57 affected members.
GSS is a prion disorder, but one that is inherited rather than acquired from eating tainted burgers from mad cows (bovine spongiform encephalopathy) or the brains of dead people (kuru). A prion is a protein that can exist in several folded forms, one of which is infectious – it makes the other forms like itself. These “rogue proteins” tend to turn brains into a spongy mess.
Prions were first described in 1954 in sheep afflicted with scrapie, but farmers had reported it since the Middle Ages. Chronicling of kuru among the Fore people of Papua New Guinea was the life’s work of D. Carleton Gajdusek, from the 1950s for many years before his legal woes. I tell the curious history of prions in chapter 4 of my essay book Discovery: Windows on the Life Sciences (Blackwell Science). (It was poorly marketed into oblivion.)
Women and children Fore infected themselves when they ate the raw brains of dead friends and relatives to honor them. The men got safe cooked parts, mostly muscle, and wives of dead warriors ate the penises, cooked I presume. You can read the gory story in Dr. Gajdusek’s Nobel speech. Then in the 1980s came the notorious stumbling bovines of England.
Because a prion is a protein, it’s encoded by a gene — the prion protein gene (PrP) on chromosome 20. Mutations at different places in the gene cause different inherited prion disorders, all of which are exceedingly rare.
 Creutzfeldt-Jakob disease (CJD) is like mad cow. Fatal familial insomnia (FFI) is an inherited prion disease that may have inspired an illness among the crew of the USS Enterprise in Star Trek: The Next Generation (“Night Terrors,” which aired March 16, 1991, Stardate 19144631.2) The ship’s wandering into a rift in space deprived crew members of dream sleep, causing terrifying hallucinations and extreme paranoia. But unlike Commander Riker and colleagues, people with FFI do not sleep at all. Nor do they recover at the end of the episode.
Creutzfeldt-Jakob disease (CJD) is like mad cow. Fatal familial insomnia (FFI) is an inherited prion disease that may have inspired an illness among the crew of the USS Enterprise in Star Trek: The Next Generation (“Night Terrors,” which aired March 16, 1991, Stardate 19144631.2) The ship’s wandering into a rift in space deprived crew members of dream sleep, causing terrifying hallucinations and extreme paranoia. But unlike Commander Riker and colleagues, people with FFI do not sleep at all. Nor do they recover at the end of the episode.
GSS progresses from memory loss and slurred speech to “prion dementia,” uncontrollable movements, limb weakness, and sometimes deafness and/or blindness. Gummy prion protein is deposited in the cerebral cortex, the basal ganglia, and especially in the cerebellum, which destroys voluntary movement.
Alice Uflacker, MD, one of the Duke researchers, describes the condition. “Typical age of onset is the 40s to 50s. Disease progression is longer than that of genetic CJD and FFI. A patient may be symptomatic for about 5 years, leading to death. Because age of onset is past young reproductive age, patients may not be aware that there is a 50% chance of passing the mutation to their offspring. Often times, however, the person at risk has contact with immediate and extended family members and has witnessed their loved ones deteriorate.”
The patient, Amanda Kalinsky, and her husband and children appeared on the front page of the New York Times on Tuesday.
Because GSS is autosomal dominant, it peppers family pedigrees in each generation, striking men and women. Even people who know their family history may have difficulty finding a physician who has heard of GSS, a problem that unites the rare disease community. Orthopedic surgeons, the specialists usually consulted when symptoms begin, look for common causes (“horses”) rather than the rare “zebras.”
Many physicians also haven’t heard of preimplantation genetic diagnosis (PGD), although it’s been around awhile. The Kalinskys learned about it from a genetic counselor, and elected to have predictive testing so Amanda could learn whether or not her father’s disease lay in her own future. It was a brave decision that few in her position for similar conditions, such as Huntington Disease, make. Now she knows she’ll develop GSS, for the disease has near-complete penetrance – inherit the mutation and you get the disease.
Amanda and her husband didn’t want her genetic fate for their children. And thanks to technology, they had a choice.
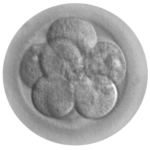 TESTING EMBRYOS
TESTING EMBRYOS
Selecting embryos isn’t new – it was first done in 1990 for X-linked mutations. In 1993 researchers selected the embryo that became Chloe O’Brien, free of the severe cystic fibrosis that affected her brother. In 1994 came another milestone, a girl conceived and selected to provide umbilical cord stem cells to treat her teenage sister’s leukemia, echoed in Jodi Picoult’s novel My Sister’s Keeper. The most famous PGDer was Adam Nash, selected to cure his sister of Fanconi anemia and born in August 2000. I vividly remember the negative vibes against this first “savior sibling” family on the Today Show; now the choice to have one child to help another isn’t so unusual.
PGD works because of a feature of the early embryos of many animal species called indeterminate cleavage. A cell can be plucked from an 8-celled embryo, tested, and the 7-celled remainder put back into a woman to continue developing, or held over for a few cell divisions. If the 7-celled embryo has the probed mutation, it can be discarded or used in research to study the genesis of the family’s disease. Some people who consider life to begin at conception object to the fate of the unused embryos. But thousands of children have been born without their family’s genetic disease thanks to PGD.
To circumvent objections to testing 8-celled embryos, researchers can use a technique called “sequential polar body analysis.” Polar bodies are by-products of egg formation that are Nature’s way to pack nutrients and organelles into a gigantic egg, prepping it to support an early embryo. The World Health Organization first suggested genetic testing of polar bodies to infer the genotype of the egg in the early 1980s. The intervention destroys the polar bodies, but they serve no function once they’ve siphoned off extra genomes and built up the egg. They’re expendable.
CHROMOSOME CHOREOGRAPHY
Although both sperm and eggs carry only one copy of the genome so fertilization can restore the double number, their timetables are markedly different. Sperm develop quickly and equally. That’s not the case for the female cells.
Technically, the female cell is called an oocyte until it’s a fertilized ovum, so there’s really no such thing as a lone ovum. Oocytes jettison parts of themselves as they form by a double division (meiosis), yielding one huge oocyte and three much smaller cells, the polar bodies. Each of these four cells houses a single genome. In fact, researchers from Harvard and Peking University have already sequenced polar body genomes to infer the genome sequence of the oocytes to which they cling. The name “polar body” is celestial and not ursine, because a polar body is like a moon that travels along with its planet.

The jettisoned polar bodies hold important clues, because as chromosome pairs part, a mutation that ends up in a polar body doesn’t end up in the all-important oocyte, or vice versa. This is the physical basis of Gregor Mendel’s observation of the segregation of traits in pea plants. So researchers can test the genes of a polar body to deduce which gene variants made it into the oocyte – in the case of Amanda Kalinsky, the GSS mutation or the normal version of the gene.
The researchers looked at the prion protein gene and 5 markers bracketing it on chromosome 20 in the polar bodies hanging onto some of Amanda’s retrieved oocytes. A different set of markers accompanied the mutant PrP gene and its normal (“wild type”) version, so they could be distinguished.

But there’s more. Female meiosis actually spawns two sets of polar bodies, at each of the two stages of the division. Examining the markers of the later-released polar bodies can reveal whether the genes on the chromosomes swap parts, called crossing over. If so, then a false negative or false positive oocyte choice could result. A paper from 2011 from Anver Kuliev and Svetlana Rechitsky (who is on the JAMA Neurology paper) reports polar body testing for 938 cycles for 146 different single-gene diseases, resulting in 345 healthy children. It works. But it isn’t really a way to avoid intervening in prenatal development.
Let’s return to the issue of timing. The first meiotic division happens when an oocyte pops out of an ovary sometime after puberty. But the second meiotic division occurs as fertilization happens. So probing polar bodies to catch those confusing crossovers, while a valid and earlier substitute for the 8-cell-stage PGD, happens at the exact time of fertilization. And as I know well from the personal name-calling that followed my recent DNA science post When Does a Human Life Begin? 17 Timepoints, many people do consider a merged sperm and egg to be a full-fledged person, equivalent to say a 53-year-old accountant. Shifting the time of selection to the very beginning of development, to a single cell, might not make a difference to them.
Anyway, the polar body technique, validated with 8-cell-stage PGD, served the Kalinskys well. The researchers injected sperm into 14 oocytes (a refinement of IVF called ICSI, for intracytoplasmic sperm injection), lost a few along the way, but the polar body testing indeed revealed a crossover event that could have led to a mistake. Three beautiful children free of GSS ultimately resulted – 3-year-old twins and a baby, shown on the front page of the New York Times.
 THE TOUGH QUESTIONS
THE TOUGH QUESTIONS
The media focused much more on gathering bioethicists for comments than on explaining polar body biology or prions, so I’ll just touch on those issues, since I teach “genethics”:
• Should an embryo be rejected because it has inherited a disease that won’t cause symptoms for half a century? That question has been raised for the BRCA genes, even more controversial because they confer susceptibility to treatable conditions.
• The slippery slope. Will we slide from preventing future people from having deadly brain diseases to choosing trivial traits? We already have. PGD is misused in sex selection.
• Are all these manipulations eugenic? Not by intent, but perhaps in consequence. Eugenics has a societal goal, and can be negative (kill the imperfect) or positive (reward the perceived best for reproducing). Medical genetics aims to alleviate suffering at the individual and family levels, but some interventions will ultimately affect the gene pool.
This landmark report on the rarest-of-the-rare Gerstmann–Sträussler–Scheinker syndrome, a unicorn among the zebras, provides some assurance that for those electing to choose embryos that have won the genetic roulette and not inherited the family’s disease, results are reliable, and can be done before the fertilized ovum divides.


There is certainly a great deal to learn about this topic.
I love all of the points you have made.